
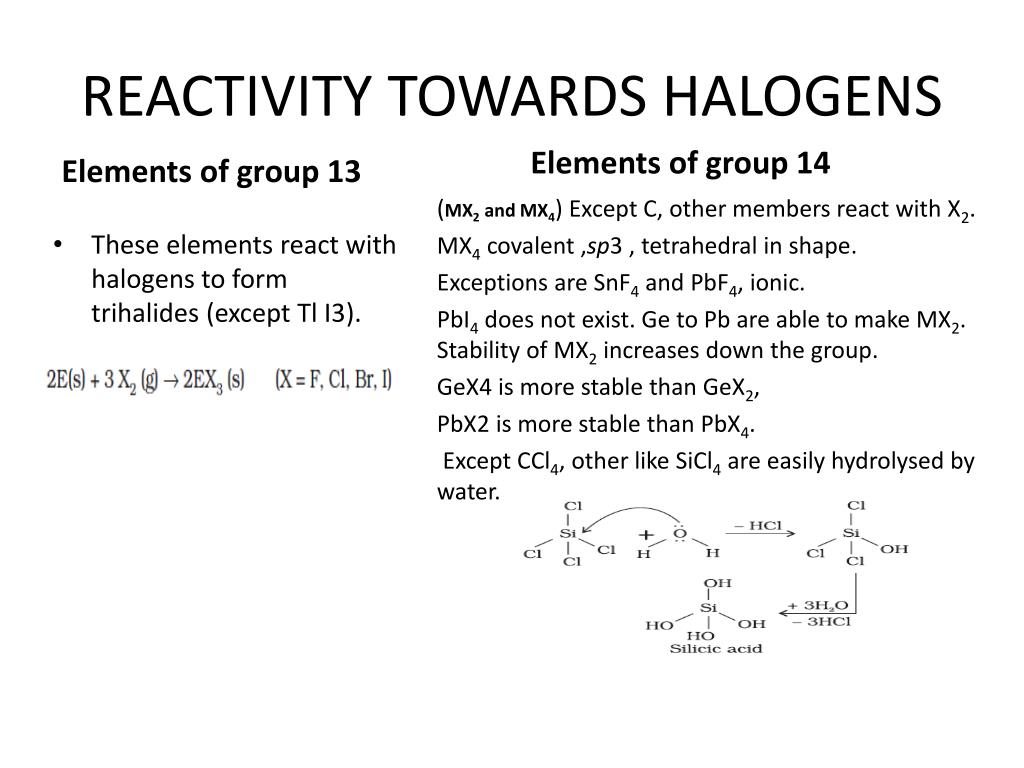
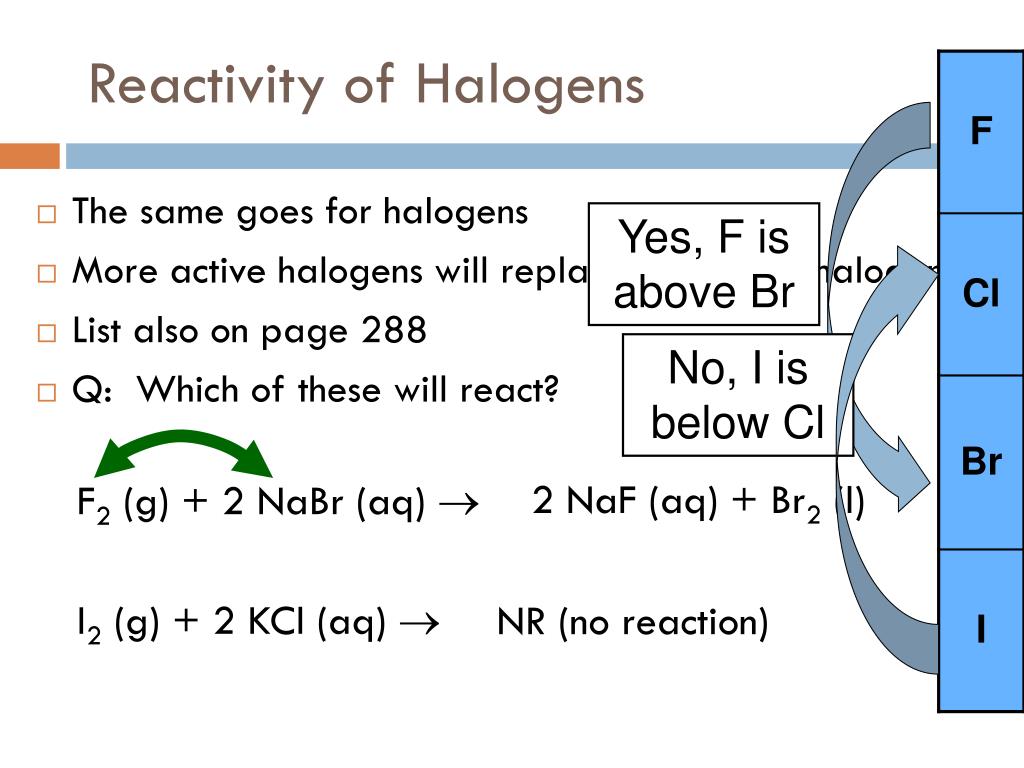
The energy of the hydrogen-halogen bond increases strongly from iodide to fluoride. The halogen elements all form compounds with hydrogen, the hydrogen halides which are: Reducing strength I - > Br - > Cl - > F - Here some example of oxidation reactions: This trend is inverted when we talk about the reducing strength of the corresponding halides. Oxidizing strength of F 2 > Cl 2 > Br 2 > I 2 As a result, there is a regular decrease in the oxidizing strength of the halogens from fluorine to iodine. On the other hand, there is a regular decrease in the first ionization energy as we go down the column. There is a regular increase in many of the properties of the halogens as we proceed down the column from fluorine to iodine, including the melting point, boiling point, the radius of the corresponding halide ion, and the density of the element. Scheme representing the trends of the halogens' properties in group 7 Some of the properties of this group of elements are shown in figure 2 belowįig 2. There is, however, a progressive change in properties from fluorine through chlorine, bromine, and iodine to astatine. Fluorine has the highest electronegativity of all elements, and they all form negatively charged ions (H -, F -, Cl -, Br -, I -, and At -). They all have very high electronegativity. As pure elements, they form diatomic molecules with atoms joined by non-polar covalent bonds. Halogens range from solid (I 2) to liquid (Br 2) to gaseous (F 2 and Cl 2) at room temperature.
Reactivity of halogens free#
The halogens exist, at room temperature, in all three states of matter:īut halogens are so reactive that they do not occur as free elements in nature Properties of the HalogensĪs mentioned above, these reactive nonmetals have seven valence electrons. This is the main reason why the halogens have the tendency to acquire an additional electron making them strong oxidizers.
Therefore, a halogen atom could get one more electron (and hold it in a p orbital), which will result in a halide ion with the same configuration than that of the noble gas next to it in the periodic table. These seven outermost electrons orbit in two different kinds of orbitals designated s (with two electrons) and p (with five). Let's think about the chlorine ion, which is usually obtained from table salt (NaCl).įig1: Periodic table of the elements showing the group 17: the halogens family.Īll halogens have 7 electrons in their outer shells, giving them an oxidation number of -1. They are non-metals, and the term "halogen" means "salt-former" because halogens react with metals to produce many important salts. Halogens are a group of elements on the periodic table found in group 17. The halogens are Fluorine (F), Chlorine (Cl), Bromine (Br), Iodine (I) and Astatine (At).They are highly reactive, therefore toxics.

They have seven valence electrons (one short of a stable octet).Halogen have very high electronegativities.Here some example of oxidation reactions:.


 0 kommentar(er)
0 kommentar(er)
